|
|
| |
|
|
| |
| |


 |

The
Project Leader's Profile
Tadashi
Suzuki (Ph.D.)
Visiting Associate Professor, Osaka
University Medical School/ Graduate
School of Medicine
Graduated from the Department of Biophysics
and Biochemistry, Faculty of Science,
University of Tokyo in 1992. Obtained
a Ph.D. degree in 1997. From 1997-2000
he was a post-doctoral fellow at the
Department of Biochemistry and Cell
Biology, State University of New York
at Stony Brook. During this period
he was a JSPS (Japan Society for the
Promotion of Science) pre/postdoctoral
fellow for young researchers (1996-1998)
and JSPS oversea research fellow (1998-2000).
In 2000, he was appointed as a Research
Assistant Professor. From December
2001, he serves as a researcher of
the Precursory Research for Embryonic
Science and Technology (PRESTO), Japan
Science and Technology Corporation
(JST). From February 2002 he was also
an RCF Assistant Professor at the Undergraduate
Program for Bioinformatics and Systems
Biology (UPBSB), Department of Biophysics
and Biochemistry, Graduate School of
Science, University of Tokyo. From
January 2004, he is appointed as a
visiting associate professor at Osaka
University Graduate School of Medicine.
 |
Project
Leader:
|
Tadashi Suzuki,
Ph.D., Visiting Associate Professor,
Osaka University Medical School/
Graduate School of Medicine |
| Research Collaborators: |
Yoko Funakoshi, Ph. D., Japan Science and Technology Agency
Kaori Tanabe, Ph. D., Post-doctoral fellow of the 21st Century COE Program of Department of Biochemistry, Osaka University Medical School / Graduate School of Medicine.
|
|


 |
Peptide:N-glycanase (PNGase) cleaves
asparagine-linked (N-linked) glycan from
glycoproteins. Recent studies have shown
that this enzyme is involved in quality contol
system for newly synthesized glycoproteins
in the endoplasmic reticulum (ER). This quality
control system distinguishes normal proteins
from misfolded and/or unassembled proteins,
that are to be degraded by the mechanism
called "ER-associated degradation" (ERAD).
There is growing evidence that impairment
the ERAD function in mammals results in various
inherited/acquired neurodegenerative diseases.
We have discovered, purified the cytoplasmic PNGase and subsequently cloned the
gene encoding this enzyme. Currently our focus is to analyze structure and function
of a newly-found protein complex that involves the cytoplasmic PNGase. It is
interesting to note that, while the monocellular organisms such as budding yeast
exhibit little effects upon defect of this enzyme, multicellular organisms showed
distinct phenotypes such as abnormal axon branching. We therefore aim to unveil
the precise role of PNGase, defect of which causes such pivotal phenotypic consequences.
Especially we are interested in how these phenotypes are related to its functional
importance in ERAD process.
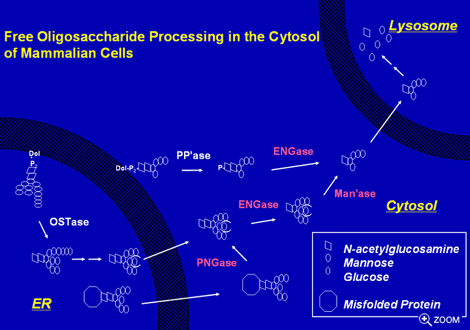
Occurrence of free N-linked glycan released by PNGase has been known for years.
It has been demonstrated in plants that free N-glycans has a hormone-like activity
promoting growth and ripening of fruits. Moreover, in mammalian cells extensive
biochemical studies revealed the very sophisticated intracellular transport/processing
pathway of free N-glycan. However, in either case the molecular mechanism of
this process remains largely unknown. For instance, recently the cytoplasmic
PNGase was found to play a major role for generating the free N-glycan in the
cytosol of budding yeast, while it was also shown that there are still unidentified,
PNGase-independent pathway to form free N-glycans.
Most recently we have succeeded in cloning the gene encoding the cytoplasmic
endo-β-N-acetylglucosaminidase (ENGase), which has been thought to be involved
in the critical step for the processing of free N-glycan in the cytosol. This
finding will lead to the better understanding of processing of free N-glycans,
which is still enigmatic even in this "post-genome" era. In the future we'd like
to figure out the whole picture of formation, modification and transport of free
N-glycans by identifying molecules involved. The molecular identification of
proteins that involved in this process will enable us to assess its functional
significance.


 |
| 1) |
Suzuki T, Hara I, Nakano M, Zhao G, Lennarz WJ, Schindelin H, Taniguchi N, Totani K, Matsuo I, and Ito Y. Site-specific labeling of cytoplasmic peptide:N-glycanase by N,N’-diacetylchitobiose-related compounds. J. Biol. Chem., 281, 22152-22160, 2006. |
| 2) |
Suzuki T, Hara I, Nakano M, Shigeta M, Nakagawa T, Kondo A, Funakoshi Y, and Taniguchi N. Man2C1, an α-mannosidase is involved in the trimming of free oligosaccharides in the cytosol. Biochem. J., 400, 33-41, 2006. |
| 3) |
Suzuki T, Park H, Kwofie MA, and Lennarz WJ. Rad23 provides a link between the Png1 Deglycosylating Enzyme and the 26S Proteasome in Yeast. J. Biol. Chem., 276, 21601-21607, 2006. |
| 4) |
Suzuki T, Park H, Hollingsworth NM, Sternglanz R, and Lennarz WJ. PNG1, a yeast gene encoding a highly conserved peptide:N-glycanase. J. Cell Biol., 149, 1039-1051, 2006. |
| 5) |
Suzuki T, Yano K, Sugimoto S, Kitajima K, Lennarz WJ, Inoue S, Inoue Y, and Emori Y. Endo-β-N-acetylglucosaminidase, an enzyme involved in the processing of free oligosaccharides in the cytosol. Proc. Natl. Acad. Sci. USA., 99, 9691-9696, 2002. |
|
|
|
|
|
| PAGE TOP |
Copyright 2003 Osaka University. All Right Reserved.
Integrated functional analyses of disease-associated sugar chains and proteins, Osaka University

|
|
|
|
|